Many supplement brands fail FDA checks not because of formulas, but because box labels miss key rules that buyers often overlook.
FDA dietary supplement labeling requirements 1 for supplement boxes focus on mandatory information display, accuracy, and placement to ensure consumer safety and regulatory compliance..
If you sell supplements in the U.S. market or work with pharmaceutical & supplement packaging, understanding box labeling rules is not optional. Keep reading below.
What information must appear on supplement boxes under FDA rules?
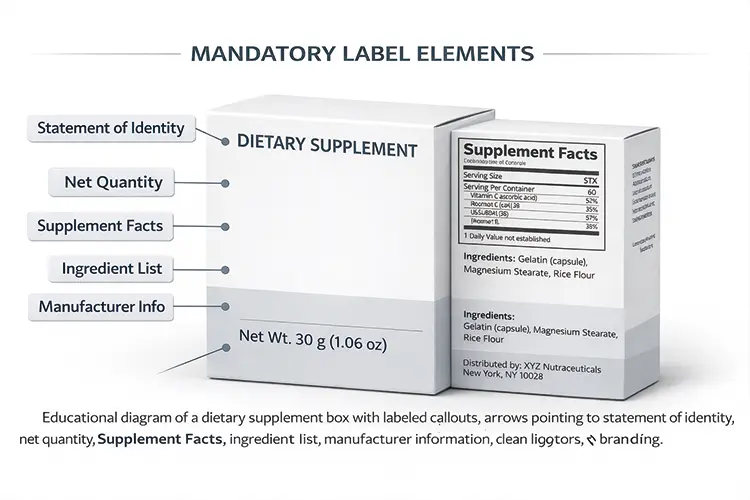
MANDATORY LABEL ELEMENTS
Many brands assume labels only matter for bottles. This mistake causes delays, relabeling, or shipment holds.
FDA requires supplement boxes to display identity, net quantity, Supplement Facts, ingredient lists 2, and responsible party information clearly and consistently.
I see this issue often when brands scale from local sales to U.S. distribution. Early on, they focus on bottle labels and treat boxes as decoration. That approach does not work once FDA or distributors review your packaging.
Core FDA-required elements for supplement boxes
The FDA treats outer boxes as part of the labeling system. If a box carries claims or product identity, it must meet the same standards as the container inside.
Required elements overview
| Label Element | Purpose | Common Factory Issue |
|---|---|---|
| Statement of Identity | Defines product type | Too small or unclear wording |
| Net Quantity | Shows amount of contents | Missing or wrong unit format |
| Supplement Facts | Nutrition disclosure | Font too small for boxes |
| Ingredient List | Transparency | Incorrect order or names |
| Manufacturer Info | Accountability | Missing U.S. address |
I have worked with Canadian and U.S. brands that had perfect formulas but failed audits because the outer box omitted net quantity or used marketing names instead of standardized ingredient terms.Many brands only fix this after switching to FDA‑ready supplement packaging boxes 3.
Placement and visibility matter
FDA does not only care what you print, but where you print it.
- Identity and net quantity must appear on the principal display panel
- Supplement Facts must be readable without opening the box
- Manufacturer or distributor details must include a valid U.S. address
From a packaging point of view, this affects dieline planning. If designers push all compliance text to side flaps to “keep it clean,” the box may fail review.
I always tell clients: compliance space is not wasted space. It protects your shipment and your brand.
How does FDA regulate font size, layout, and readability on boxes?
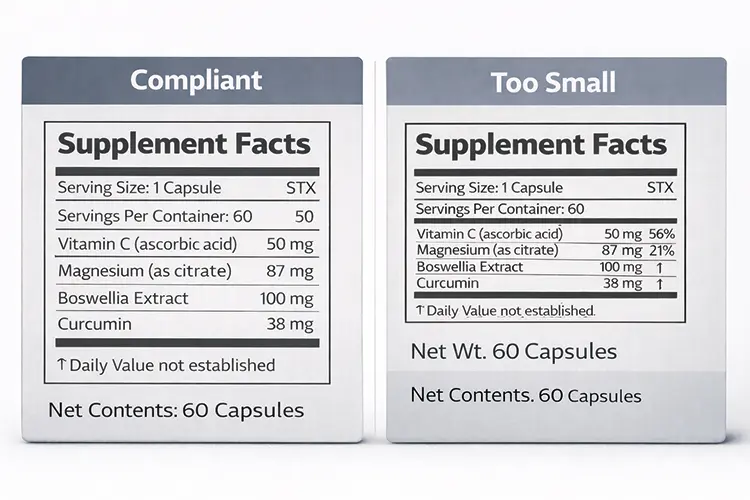
FONT AND LAYOUT RULES
Many supplement boxes fail not because information is missing, but because it is hard to read in real life.
FDA labeling rules set minimum type sizes and basic layout standards 4 so that key information on supplement boxes stays legible and easy to find.
In factory reviews, this is one of the most common reasons for redesign and reprints.
Minimum font size in simple terms
FDA treats different text areas on supplement boxes differently.
The net quantity statement is regulated by character height, while the Supplement Facts panel is regulated by point size.
To make these rules easier to apply in real projects, the requirements can be summarized as follows.
FDA Minimum Font Size Requirements (Practical Use)
| Text Area | Measurement Method | Minimum Requirement | Notes |
|---|---|---|---|
| Net quantity statement (PDP) | Character height (inches) | ≥ 1/16 inch for small panels ≥ 1/8 inch for larger panels | Height is measured by the lowercase “o,” not total line height |
| Supplement Facts — main text | Point size | ≥ 8-point | Default rule for most packages |
| Supplement Facts — heading | Relative size | Larger than body text | Must be bold and visually prominent |
| Column headings / footnotes | Point size | ~6-point | Allowed, but must remain legible after printing |
| Small / intermediate package exception | Point size | ~4.5–6 point | Exception only; requires qualifying package size |
For design and production teams, the working principle is straightforward:
- Use 8-point or larger type for Supplement Factson custom supplement packaging boxes unless the package clearly qualifies for a size exception.
- Size the net quantity statement so the character height comfortably exceeds the 1/16–1/8 inch range for the panel.
- If smaller sizes are planned, confirm FDA “small” or “intermediate” package status before final artwork approval.
Layout consistency rules
Beyond font size, FDA also evaluates how information is structured on the box.
- The Supplement Facts panel must follow FDA’s required order and standardized formatting.
- The “Supplement Facts” heading must be bold and more prominent than other text.
- Compliance text should use simple, non-decorative fonts, not script or highly stylized type.
- Line spacing and white space should keep the panel easy to scan, not crowded.
From a production standpoint, these rules directly affect dielines, artwork, plates, and proofs.
Text that appears “just readable” on screen often becomes thinner and lighter once printed, especially after coatings, laminations, or when using textured stocks.
Practical factory advice
In real production, most reprints happen because readability is not tested under real conditions.
The following checklist helps reduce that risk.
Factory Checklist to Prevent Readability Reprints
| Checkpoint | Purpose |
|---|---|
| Review a full-size physical proof | Screens do not reflect real print behavior |
| Check under retail and warehouse lighting | Studio lighting can hide contrast issues |
| Maintain strong text–background contrast | Thin light text on dark backgrounds fails quickly |
| Keep safe margins around compliance text | Prevent die-cut shifts from damaging text |
| Review after coating or lamination | Finishing often reduces clarity |
Boxes are handled, stacked, and scuffed over time.
Readability must survive printing, transport, and real-world shelf life — not just pass a digital mockup.
Can marketing claims on supplement boxes cause FDA violations?
CLAIM RISK ZONES
This is where most brands get into serious trouble.
FDA strictly limits health and disease-related claims 5 on supplement packaging, including outer boxes.
I have seen shipments held because of one sentence printed on the box front.
Types of claims FDA monitors closely
| Claim Type | Risk Level | Example |
|---|---|---|
| Disease Claims | Very High | “Treats arthritis” |
| Structure/Function | Medium | “Supports joint health” |
| General Wellness | Low | “Daily nutrition support” |
Boxes often carry stronger language than bottles because they act as shelf marketing. FDA does not allow this difference.
Why boxes are high-risk
- Boxes are more visible than bottles
- Retailers rely on outer packaging for review
- Claims on boxes must match claims on containers
In one case, a brand used compliant wording on bottles but added aggressive claims on the box for retail appeal. The entire packaging set had to be scrapped.
Safe design strategy
I advise brands to:
- Align all claims across box, bottle, and inserts
- Keep claim language conservative
- Let design and structure communicate premium, not words
Strong structure, rigid boxes, and clean layouts signal quality without regulatory risk.
Do supplement boxes need FDA compliance if products are sold online only?
ONLINE VS PHYSICAL SALES
This is a common misunderstanding.
FDA labeling rules apply to supplement boxes even for online-only brands if physical packaging exists.
Selling online does not remove packaging obligations.
Key points many brands miss
- FDA regulates labeling, not sales channels— even for online-only supplement brands using custom boxes.
- If a product is shipped in a box with printed claims, it counts
- Warehouses, fulfillment centers, and customs inspections still apply
From a packaging factory view, I see many DTC brands start simple. Later, when they enter Amazon FBA or retail, old packaging becomes unusable.
Cost impact of ignoring this early
| Scenario | Cost Result |
|---|---|
| Redesign after launch | High |
| Reprint boxes | Very High |
| Delayed retail entry | Lost revenue |
| Compliance-first design | Lowest long-term cost |
I always suggest designing boxes with FDA compliance from day one, even for small batches. Low MOQ printing makes this possible without heavy upfront risk.
Conclusion
FDA-compliant supplement boxes protect your shipment, your brand, and your ability to scale in the U.S. market.
-
See the official FDA rules for dietary supplement labeling and carton requirements. ↩
-
Check an authoritative checklist of all mandatory statements for supplement labels. ↩
-
Explore real examples of custom supplement boxes designed to meet FDA labeling rules. ↩
-
View official FDA specs for Supplement Facts font sizes and required label layout. ↩
-
Learn how FDA distinguishes allowed structure/function claims from prohibited disease claims. ↩